New Publication: Self-Testing for Respiratory Viruses
On 3 September 2025, the pilot study “Self-testing for 5 respiratory viruses in adult VACCELERATE volunteers in Germany” was published in Frontiers in Public Health. The study explored the use of rapid antigen tests to simultaneously detect SARS-CoV-2, Influenza A and B, Respiratory Syncytial Virus (RSV), and Adenovirus in adults in Germany. The results highlight that self-testing can be a valuable tool for monitoring community-acquired respiratory infections.
Rapid Test Study MAK5-50+
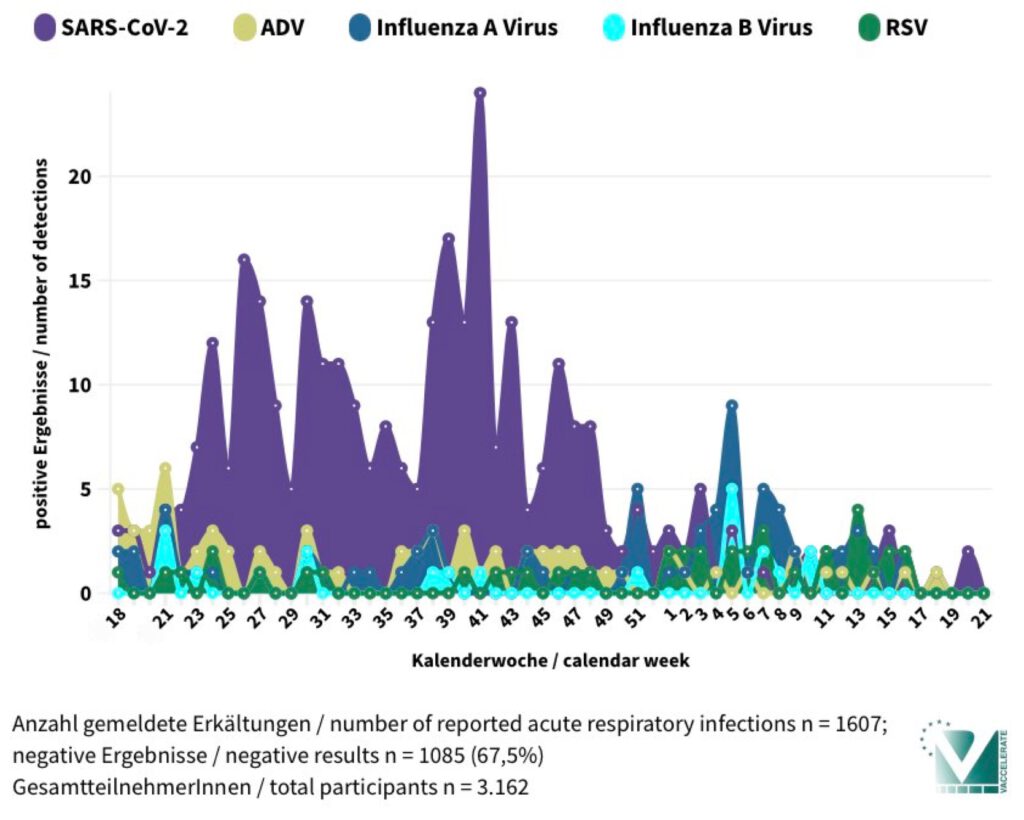
Am 25. Mai 2025 endete die einjährige Laufzeit unserer deutschlandweiten Studie MAK5-50+. Mehr als 3.100 Teilnehmende ab 50 Jahren haben mithilfe eines speziellen Schnelltests getestet, welche Viren bei Erkältungssymptomen nachgewiesen wurden.
In einem Webinar wurden die zentralen Ergebnisse, die Methodik der Studie sowie wichtige gesundheitsrelevante Erkenntnisse vorgestellt. Zur Aufzeichnung des Webinars: https://youtu.be/9XE2N1wjzLQ
Was wurde untersucht?
Ziel der Studie war es, herauszufinden, welche Atemwegsviren aktuell in Deutschland zirkulieren und wie häufig sie auftreten – speziell bei Menschen über 50 Jahren. Die von uns eingesetzten Schnelltests – ähnlich wie bekannte Corona-Selbsttests – konnten gleich fünf verschiedene Erreger (SARS-CoV-2, Influenza A und B Virus, ADV und RSV) detektieren. Die Tests wurden per Post verschickt, die Ergebnisse bequem online eingetragen.
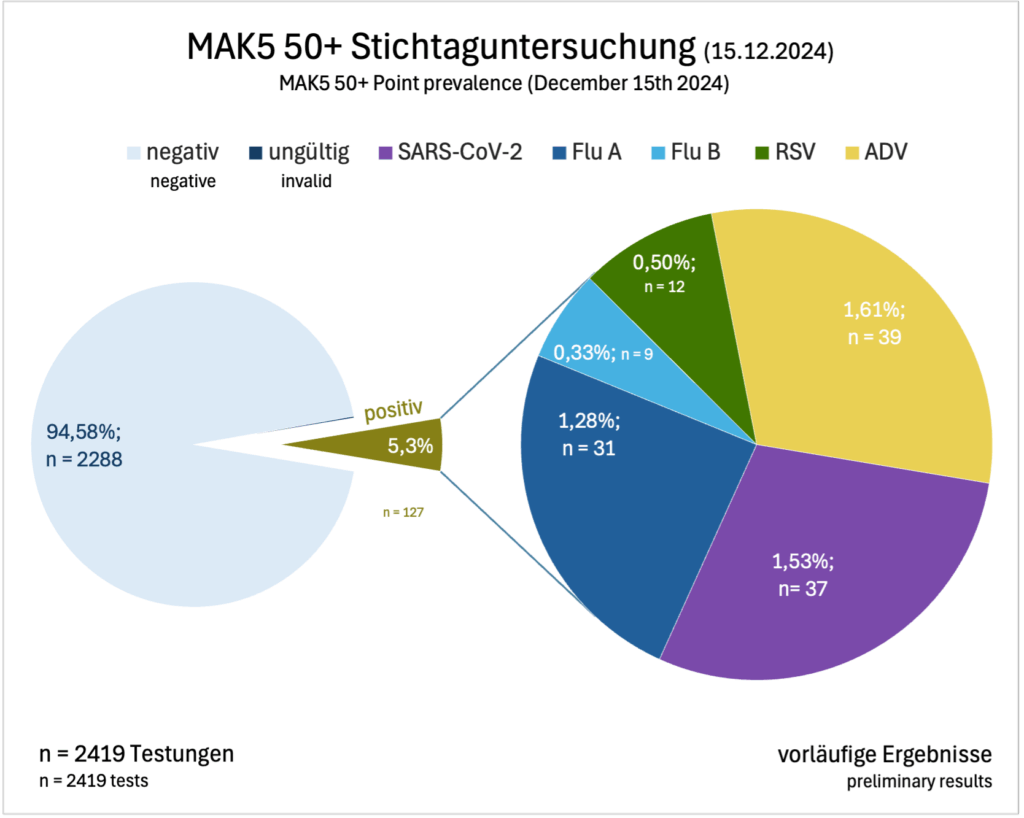
Besonders spannend: Bei einer Stichtagsuntersuchung am 15.12.2025 meldeten 2.419 Teilnehmende (76,5 %) ihre Testergebnisse. Der am häufigsten nachgewiesene Erreger war ADV (n = 39), gefolgt von SARS-CoV-2 (n = 37) und Influenza A Virus (n = 31).
Wenn Sie künftig über neue Studien und Teilnahmemöglichkeiten informiert werden möchten, tragen Sie sich gerne in unser Freiwilligenregister ein: https://v-reg.eu/
On May 25, 2025, the one-year duration of our nationwide study MAK5-50+ in Germany came to an end. More than 3,100 participants aged 50 and over used a special rapid test to identify which viruses were present during episodes of cold-like symptoms.
The main results, the study methodology, and key health-related findings were presented in a webinar. Watch the recording here: https://youtu.be/9XE2N1wjzLQ
What was investigated?
The goal of the study was to determine which respiratory viruses are currently circulating in Germany and how frequently they occur—specifically among people over the age of 50. The rapid tests we used—similar to common COVID-19 self-tests—were able to detect five different pathogens (SARS-CoV-2, Influenza A and B viruses, ADV, and RSV). The tests were sent by mail, and participants conveniently submitted their results online.
Particularly interesting: In a point prevalence on December 15, 2025, 2,419 participants (76.5%) reported their test results. The most frequently detected virus was ADV (n = 39), followed by SARS-CoV-2 (n = 37) and Influenza A virus (n = 31).
If you would like to be informed about future studies and participation opportunities, feel free to sign up for our volunteer registry:
https://v-reg.eu/
What is VACCELERATE?
Questions? Remarks? Contact us: info@vaccelerate.eu.

Social media updates after 27 January 2025 on https://bsky.app/profile/olivercornely.bsky.social and https://www.linkedin.com/in/oliver-a-cornely-7859b747/.
Continued VACCELERATE Infrastructure
VACCELERATE Study Nurse Course
Find out more about this VACCELERATE course providing the needed expertise to conduct a clinical trial
Volunteer Registry
> 107.000 Volunteers
We have set up a database in which you can register if you are interested in participating in a clinical study.
Clinical Trial Sites
525 Sites
The VACCELERATE Site Network is a platform for experienced clinical trial sites interested in conducting (vaccine) studies. You can register by answering a short questionnaire.
Partners
Project updates between February 2021 and January 2025:





































