Laboratory Site Capacity Building

Work package objectives:
The overall goal is to provide ‘state-of-the-art’ laboratory expertise for all vaccine clinical trials in VACCELERATE by building and maintaining a fully operational laboratory network with sites that can be selected for clinical studies with an external quality assessment. Tapping into LAB-Net, an IMI-funded infrastructure within the COMBACTE projects, VACCELERATE will provide access to a Europe-wide network of more than 800 laboratories in 41 countries. The team develops, validates and deploys detection assays for circulating and new SARS-CoV-variants during the clinical vaccination studies and validates and deploys next-generation tests. It provides together with WP5 and WP8 study-specific training to sites selected to participate in clinical studies and assists in finalising clinical protocols by adjusting SOPs, in line with the study objectives for standardised and harmonised sampling. The goal is to perform targeted and whole genome sequencing determination from specimens from vaccinated individuals developing respiratory symptoms. This provides information about evolution of the virus and enables identification of potential virulence markers, and emergence of markers of resistance to vaccination. This work will expand the existing PREPARE and RECOVER biobank with patient informed consent, well-characterised clinical samples collected in clinical vaccine trials for use in further studies.
WP Lead:
Prof. Surbhi Malhotra-Kumar
University of Antwerp, Laboratory of Medical Microbiology, Vaccine and Infectious Disease Institute, Antwerp, Belgium
Work Package Results
All public documents can be shared upon request. Please contact: info@vaccelerate.eu.
Standard Operating Procedures (SOPs)
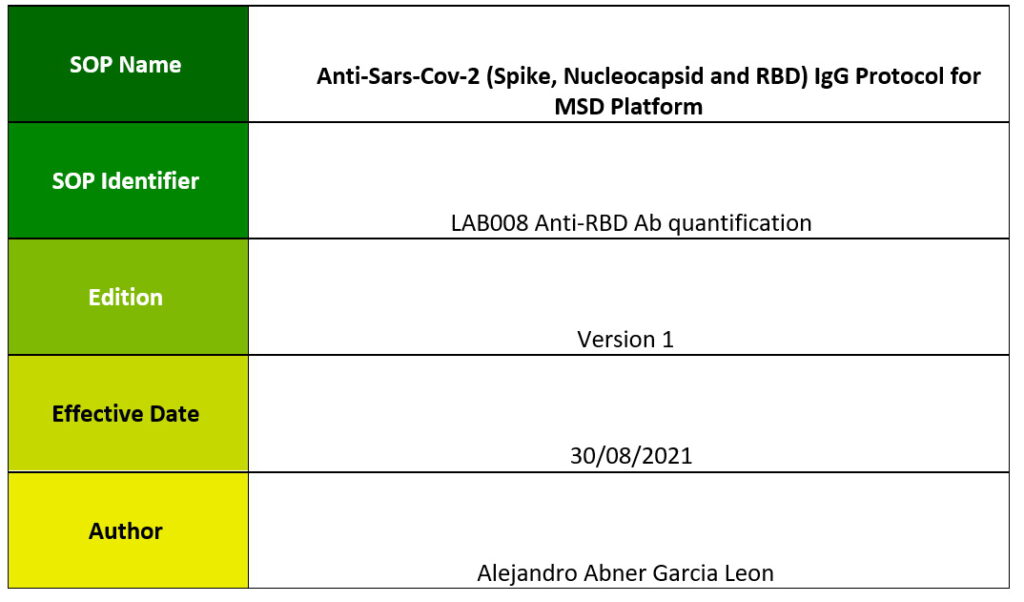
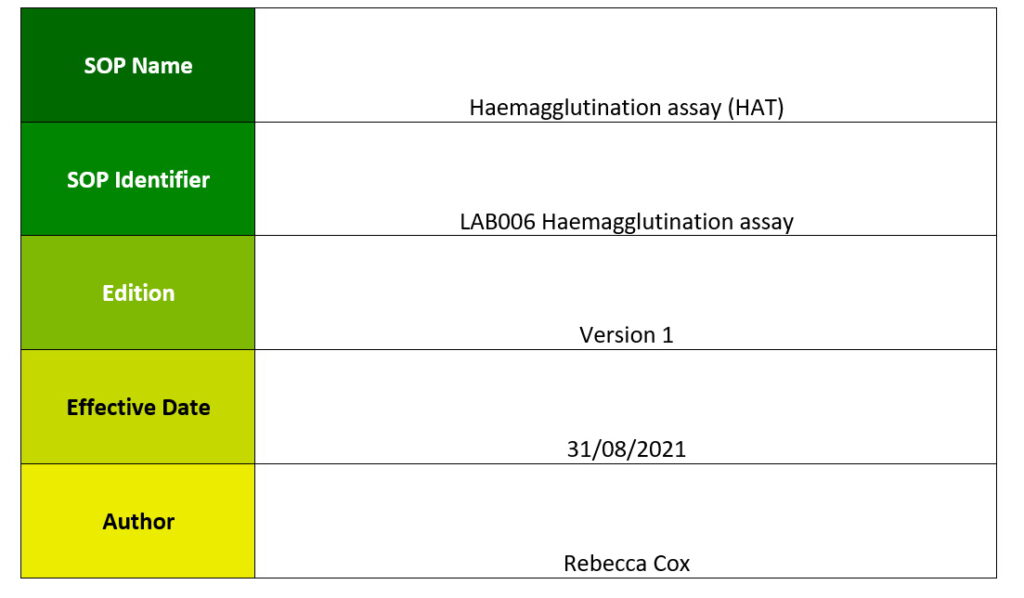
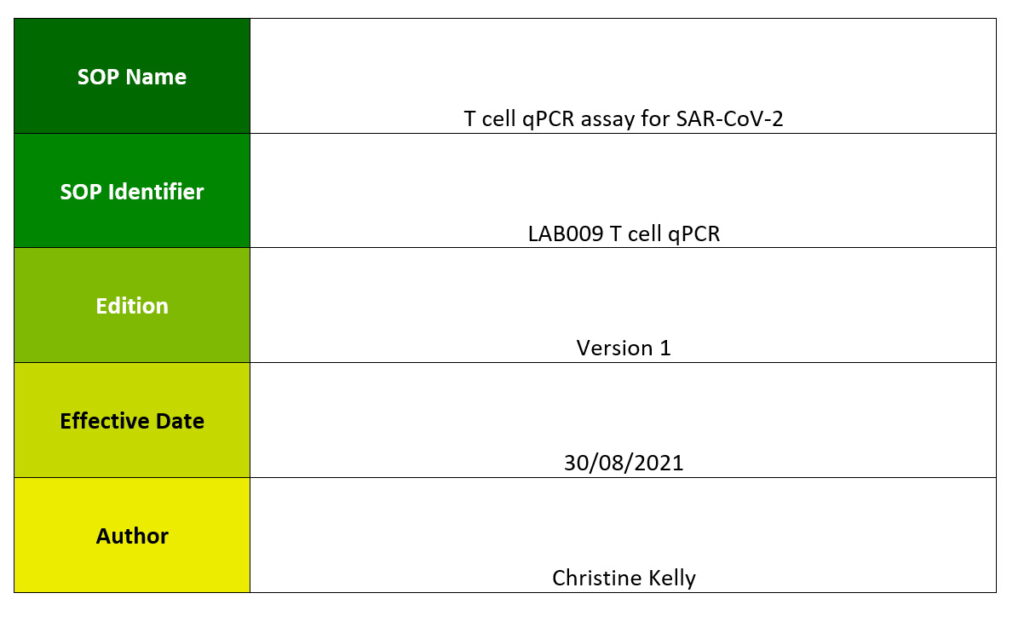
One aim of WP6 is to produce a standard set of Standard Operating Procedures (SOPs) that allow harmonisation of the laboratory procedures used in the VACCELERATE network to minimise the variation of the analytical assessments, standardising the measurements to facilitate the head-to-head comparison across different laboratories.
In the harmonization process of the laboratory assays, rigorous quality control (QC) and validation parameters are implemented to ensure the robustness, reproducibility, specificity, and sensitivity. Including the utilization of WHO/BIBSC biological controls/standards, which will allow the results to be expressed in International Standard Units where applicable.

Protocols for rapid deployment of collection and transport of specimens in line with the study objectives
Lab support strategy to harmonise lab assays in the network
The aim of this document is to produce a standard set of SOPs that allow harmonisation of the laboratory procedures used in the VACCELERATE network to minimise the variation of the analytical assessments, standardising the measurements to facilitate the head-to-head comparison across different laboratories.