Public Health Needs

Work package objectives:
The goal of WP7 is to establish 1) a living document of completed, running, and planned clinical trials evaluating COVID-19 vaccines and 2) a living systematic review (with meta-analysis) of identified completed COVID-19 vaccine trials. The team will conduct a living monitoring of trial quality and transparency with a feedback to investigators and the community to improve future trials. It will additionally identify gaps in public health knowledge on COVID-19 vaccines and vaccination in general based on the systematic review and stakeholder involvement. By developing phase 2 & 3 master protocol templates based on identified gaps and harmonisation results, the work of WP7 will facilitate discussions on harmonisation of COVID-19 vaccine clinical trials.
WP Lead:
Prof. Antonio Javier Carcas Sansuán
Clinical Pharmacology, La Paz University Hospital- Idipaz & Department of Pharmacology, School of Medicine, Universidad Autonoma de Madrid, Spain
Work Package Results
All public documents can be shared upon request. Please contact: info@vaccelerate.eu.
Report on unanswered COVID-19 vaccines and vaccination priority questions for future trials
Identifying and filling public health knowledge gaps in COVID-19 vaccine development has been deemed one of the key network objectives and included as one of the work streams within VACCELERATE. This work is based on close collaboration with other work streams within VACCELERATE, general public outreach and is anchored in a broad European and international dialogue about new COVID-19 vaccine clinical trials aiming to inform both ongoing and future studies through a living document of priority questions that reflects knowledge gaps and emerging priorities from the public health perspective.
In spring 2021, a stakeholder survey helped us identify a list of emerging and unanswered public health and clinical research questions in relation to vaccine safety, efficacy, vaccination schemes. A total of 89 responses to the stakeholder survey have been received, resulting in 148 submitted priority topics. Responses have been received from 23 countries across the EU but also associated countries via clinical sites registered in the EUVAP platform.
The survey replies have been grouped and compiled into a final list of 17 priority questions that are listed below.
From these findings 3 top public health priority questions for future COVID-19 trials have been identified in November 2021.
The survey is repeated regularly.
Further information and the full report can be found here:
https://chip.dk/Research/Studies/VACCELERATE/Report
https://www.chip-crf.info/redcap/surveys/?s=49RYXRWXTY
https://chip.dk/Portals/0/files/VACCELERATE/VACCELERATE_WP7_D7.6_Report_V1.0w_appendixes.pdf
PRIORITY QUESTIONS IDENTIFIED BY VACCELERATE SURVEY
Vaccine administration
- How can immunisation schedule (booster timing and number) and technologies (vaccine dose and type) be optimised to ensure maximum protection?
- What is the comparative advantage of heterologous vs. homogenic vaccination in terms of efficacy, safety and duration of protection?
- Can novel vaccines achieve non-inferiority efficacy and safety by non-parenteral route and possibly with only one dose?
- What should the vaccination strategy be for recovered patients?
Vaccination in immunocompromised
- What is the vaccine efficacy and are there other immunological correlates of protection than antibodies in various immunocompromised groups?
- How can immunisation schedule (booster timing and number) and technologies (vaccine dose and type) be optimised for the immunocompromised group to ensure maximum protection?
Pediatric vaccination
- What is the efficacy and the specific immune response to the vaccine in children, including immunocompromised pediatric population?
- What are the long-term safety considerations of vaccination in children?
- What is the relationship in terms of protection between vaccination and immuno-mediated diseases such as MIS-C?
Long-term protection and immunity
- How long does immunity (humoral-cellular) last after vaccination with current vaccines?
- Are currently available vaccines effective against SARS-CoV-2 variants in the short- and long-term and is there a need to develop new vaccine to protect against the VOCs?
- What is the best measure of protective immunity after vaccination at the individual level and when after vaccination should it be taken?
Protection against new variants
- Are currently available vaccines effective against SARS-CoV-2 variants in the short- and long-term and is there a need to develop new vaccine to protect against the VOCs?
Long-term vaccine safety/side effects
- What are the long-term adverse side effects of vaccination in terms of vaccine-related or vaccine-induced diseases (autoimmune, oncologic, fertility etc.)?
Infection transmission prevention
- Are current vaccines and vaccine strategies effective in preventing SARS-CoV-2 transmission?
Public health/policy
- How can awareness of and confidence in vaccine programmes be improved to address vaccine hesitancy and misinformation with focus on specific population groups?
- How can wide-scale global vaccination coverage be ensured within reasonable timelines, especially in resource-limited settings?
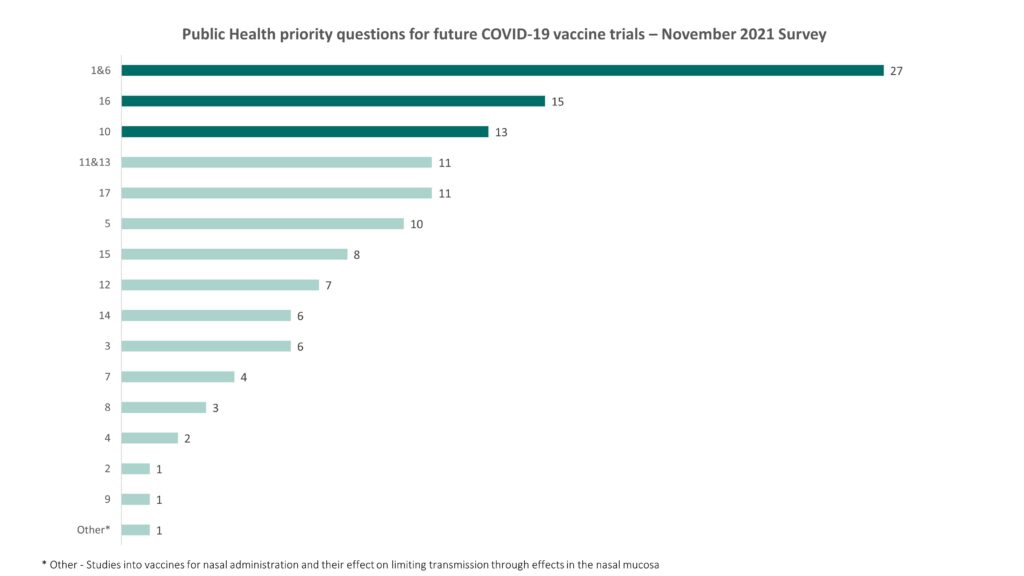
In November 2021 a new survey was circulated among the VACCELERATE Consortium focused on the revision of the initial list of priority questions identified in D7.4 First report on unanswered COVID-19 vaccines and vaccination priority questions for future trials was finalised in September 2021.
The survey aimed to rank the list of the 17 priority questions and identify the top 3 most important unanswered questions for potential upcoming COVID-19 vaccines clinical trials to address as well as collect any new emerging questions. Below you can find the list of the top 3 priority questions identified by the survey respondents.
1. How can immunisation schedule (booster timing and number) and technologies (vaccine dose and type) be optimised to ensure maximum protection (incl. immunocompromised groups)?
2. How can awareness of and confidence in vaccine programmes be improved to address vaccine hesitancy and misinformation with focus on specific population groups?
3. How long does immunity (humoral-cellular) last after vaccination with current vaccines?

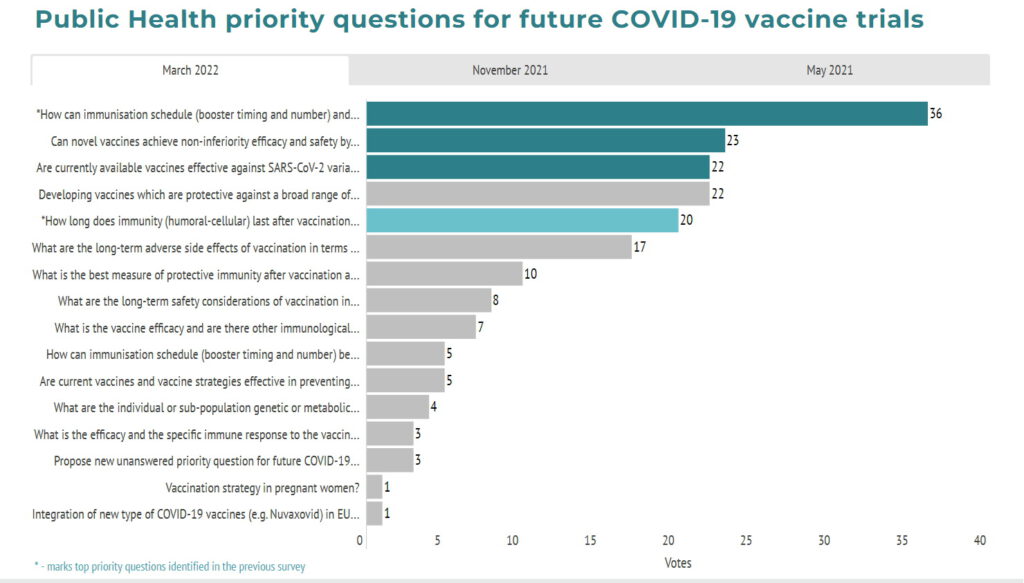
Proposed new priority questions for future COVID-19 vaccine studies (March 2022)


REPORT UPDATES HERE: https://chip.dk/Research/Studies/VACCELERATE/Report
List of standardised data for vaccine trial protocol design
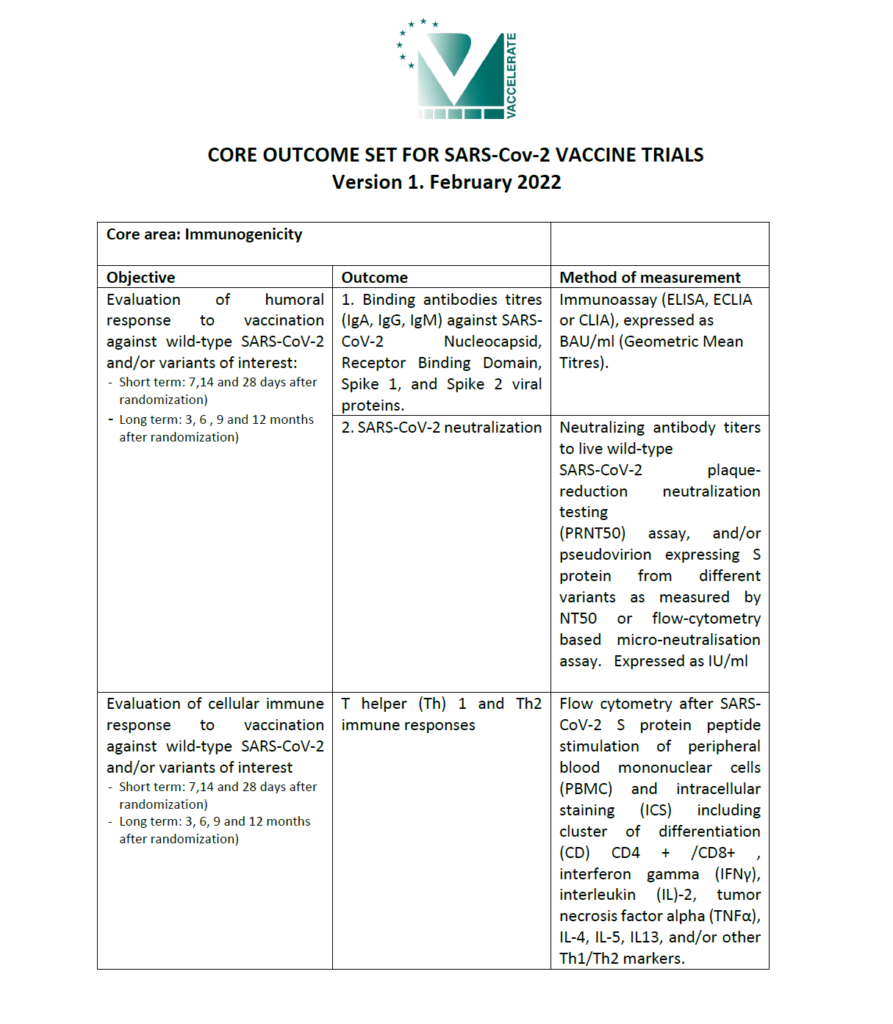
A Core Outcome Set is defined as “an agreed standardised collection of outcomes that should be measured and reported for a specific area of health”. In general lines, COS are established to ensure that critically important outcomes are systematically and consistently reported in all clinical trials.
Considering the aims of VACCELERATE, it is essential to adopt a standard set of clinical endpoints for vaccine evaluation across all phase II and phase III trials in order to provide uniform, comprehensive evaluations of benefit and risk and to support pooling data for analyses of immunologic surrogate endpoints and hard efficacy outcomes.