Volunteer Registries

Work package objectives:
The overall objective of WP10 is the design and implementation of an EU-wide, dynamic, harmonised, and sustainable Volunteer Registry for phase 2 & 3 vaccines clinical trials with an initial focus on the COVID-19 pandemic, and for the future expansion to any forthcoming European epidemic/pandemic. It will be built on the recently established German Volunteer Registry and is currently expanding to four other countries, with 12 other countries on the way. WP10 strives to expand volunteer registries from healthy individuals to patients in specific sub-groups which is an essential resource for the identification of eligible volunteers for phase 2 & 3 vaccine clinical trials and can also be used to facilitate access to populations underrepresented in current vaccine trials. The added values of VACCELERATE for vaccine developers lie in the specific expertise needed for vaccine trials, combined with access to volunteers with or without co-morbidities, in- and out-patient settings, and the capacity to enrol a high number of participants in a short period of time.
WP Lead:
Zoi Dorothea Pana, MD, MSc, PhD
Lecturer in Pediatrics, Subspecialty Epidemiology, Infection Control and Prevention (EUC, CY), European University of Cyprus (EUC); Ministry of Health (CY), Cyprus
Work Package Results
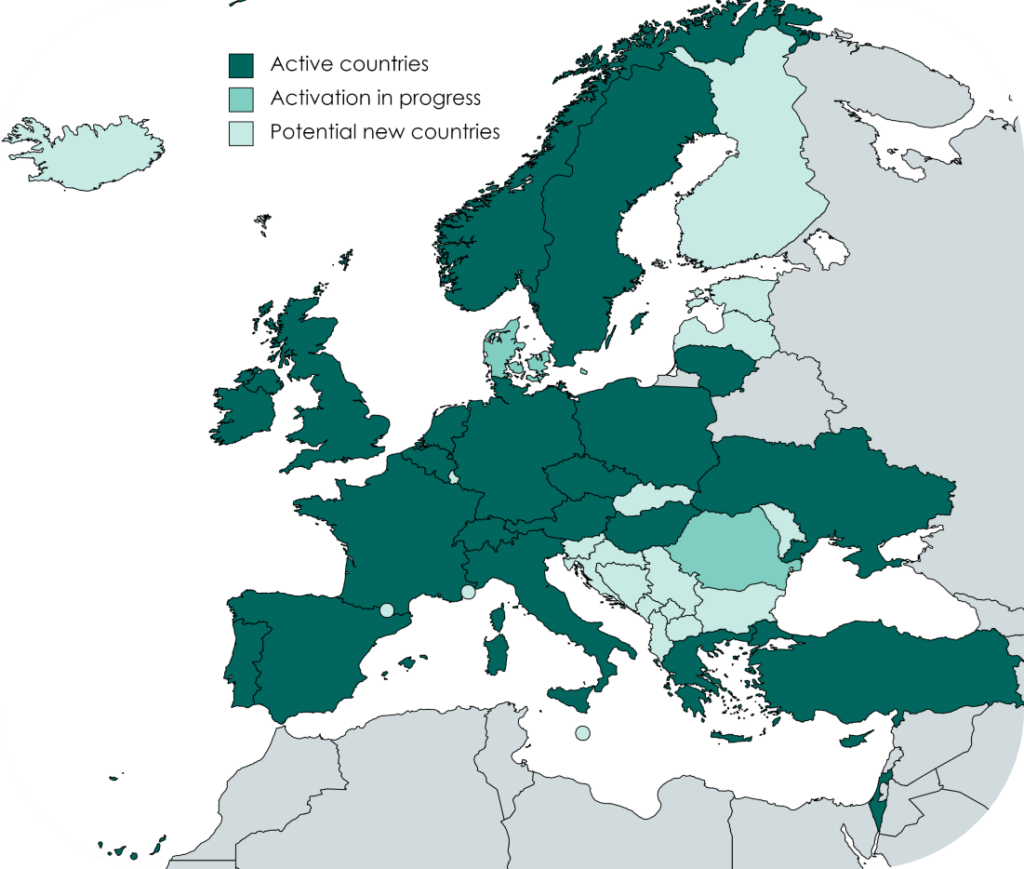
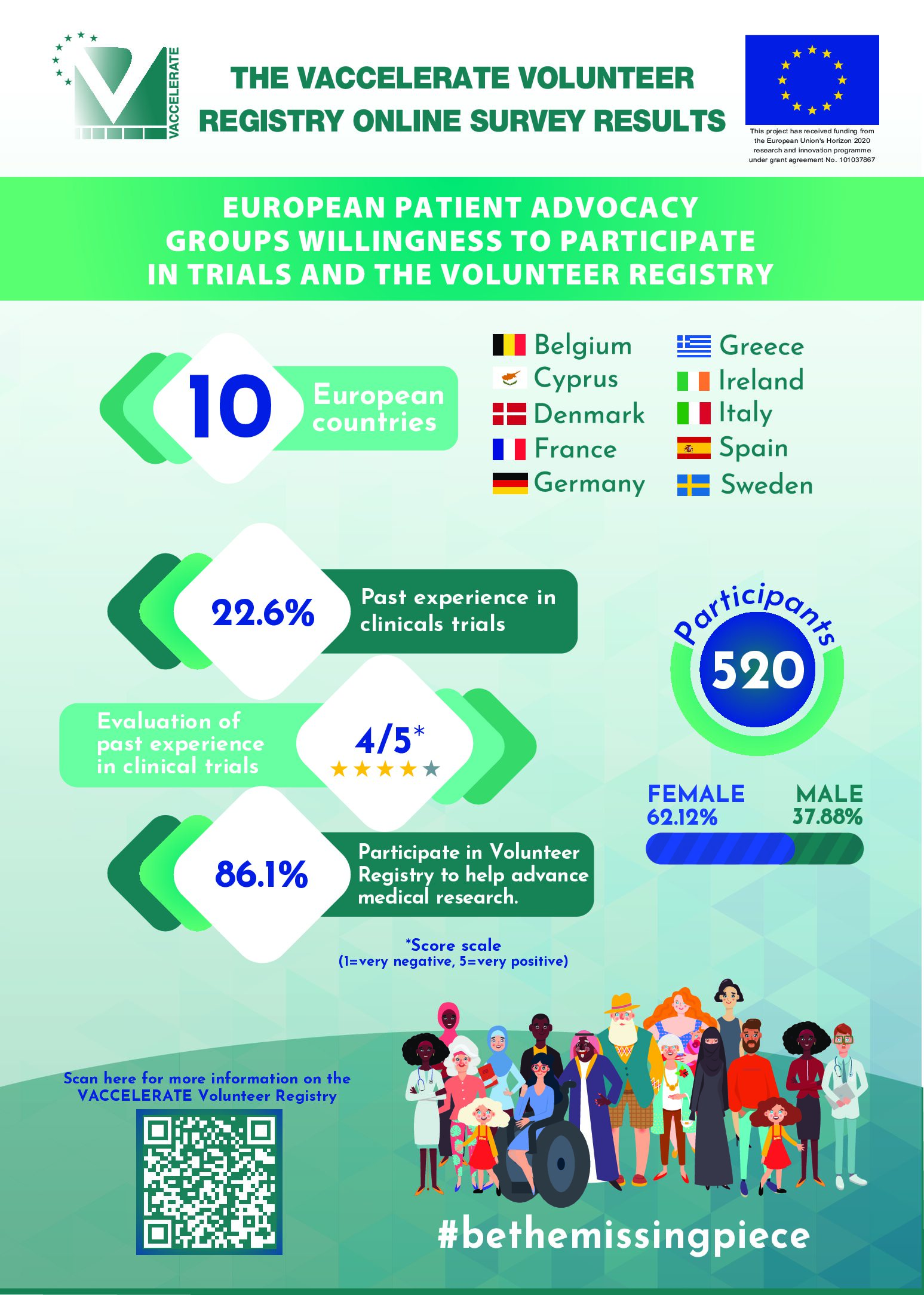
The Volunteer Registry is available in 22 countries and in several local languages. More countries are joining. More than 100,000 volunteers already registered.
More information and registration on the Volunteer Registry Website.
Work Package 10 developed a large variety of promotional materials including posters, brochures, flyers, videos, educational cards and puzzles for children. All of these were translated to different languages. You can find all materials in all languages on the website of the VACCELERATE Volunteer Registry.
Videos
You can watch the videos created by WP10 here: https://vaccelerate.eu/videos/. More videos on VACCELERATE topics can be found on our YouTube channel. You can download the promotional material here: https://www.dropbox.com/sh/hfaom2vslyqa1nh/AACRgKHKhcxlM0xVvASMvAQ9a?dl=0.