Data Management, Standards & Sharing

Work package objectives:
WP9 aims to develop a data management toolbox to be able to propose standardised data management services, promoting interoperability of data across vaccine trials using the VACCELERATE network. This maximises the speed and efficiency of study setup, for example by promoting a standardised set of outcomes, data items and data collection instruments. It additionally maximises the efficiency of data collection, promoting data collection protocols that can be shared between different vaccine trials, using a single data collection system. The team aims to maximise the ‘FAIRness’ and thus the scientific value of the generated data, by 1) ensuring it is well described by metadata, 2) promoting the deposition of data and documents into repositories, and 3) promoting de-identification and other data preparation steps, as well as 4) ensuring data is as inter-operable as possible.
WP Lead:
Dr. Jacques Demotes
Director General of ECRIN, European Clinical Research Infrastructure Network (ECRIN), France
Work Package Results
All public documents can be shared upon request. Please contact: info@vaccelerate.eu.
Feasibility assessment of using data standards for vaccine trials
VACCELERATE data management and data sharing plan (DMP)
The objective of this report is to describe the issues around using data standards within the various clinical studies in the VACCELERATE project, with the focus on assessing the feasibility of such an approach.
The first part of the report describes the different types of data standards and then examines the most important standards in use within clinical trials. The second part is focused on the VACCELERATE project.
Report on use of interoperable data points and common collection protocols/systems
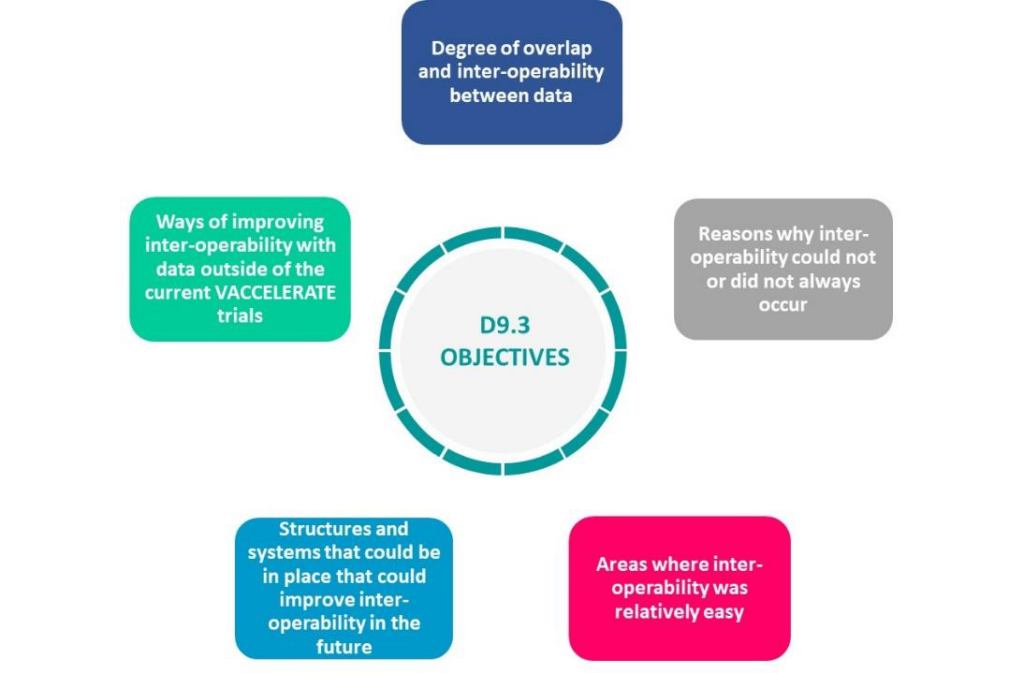
The objective of this report is to define degree of commonality between the clinical trial metadata, protocols and the data collection systems/items in VACCELERATE project and to explore the degree of overlap and inter-operability between data, reasons why inter-operability could not or did not always occur, areas where inter-operability was relatively easy and the structures and systems that could be in place that could improve inter-operability in the future and for the data outside of the current VACCELERATE trials.
This report also provides suggestions on how to improve interoperability, ensure harmonisation, standardization of the protocols, metadata, and the data management systems for future VACCELARATE and vaccine clinical trials in general.