
VACCELERATE Site Network
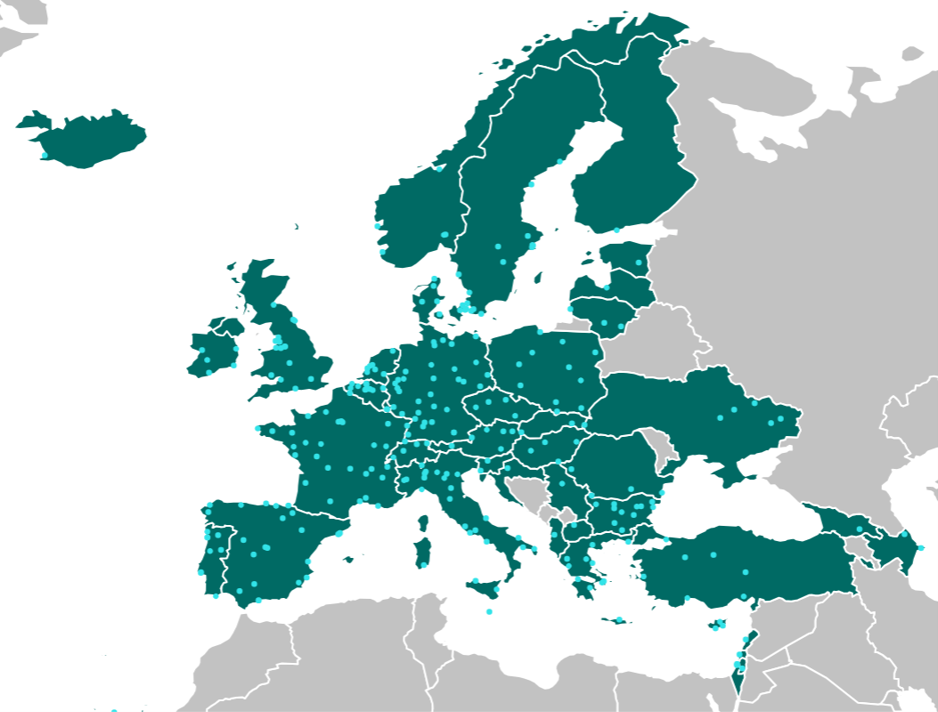
525 clinical trial sites from 57 countries have joined the Site Network so far. The majority is either located in the EU or associated with the H2020 programme.
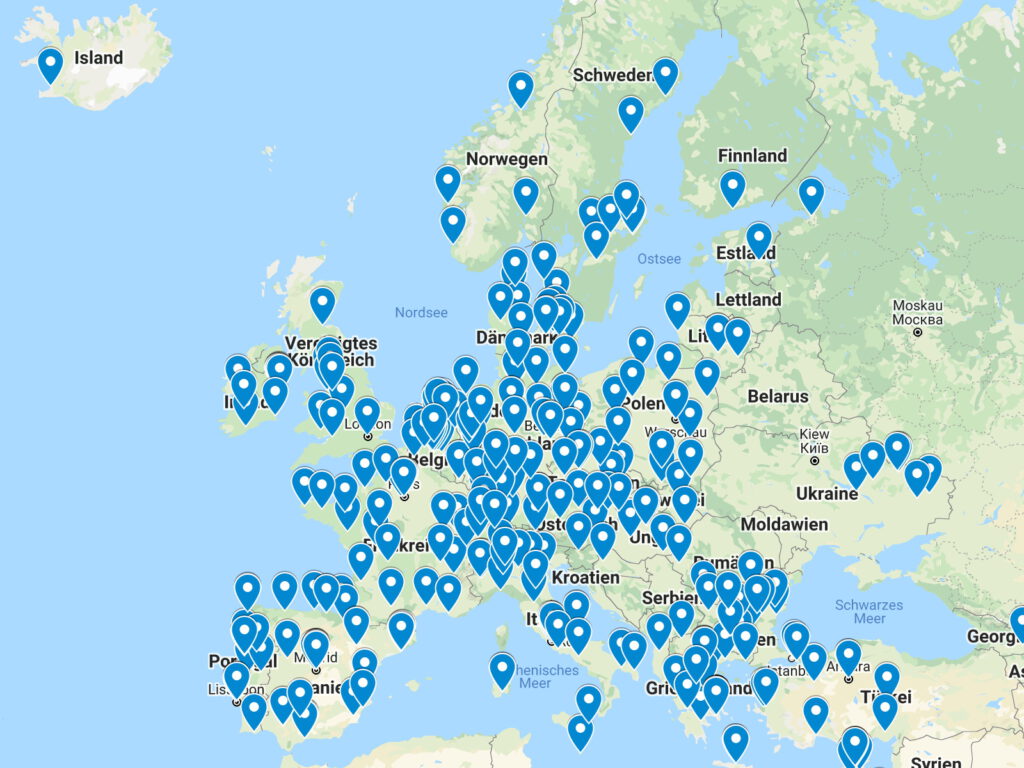
VACCELERATE Site Network laboratories
REGISTER NOW!

Become one of our more than 500 registered trial sites and register today!
All you need to do is send an email to trialsites@vaccelerate.eu including:
- Name of your site
- Postal address
- Full name of a contact person
- Contact email address


VIDEO on the VACCELERATE Site Network:

VACCELERATE Clinical Studies
Clinical trials serve the purpose of clarifying open questions regarding new vaccines, therapies or medicines. By participating in clinical trials, you can make a major contribution to scientific progress. As a study participant you support the research of diseases and the development of new treatment options and at the same time receive comprehensive medical care.
VACCELERATE is carrying out three COVID-19 vaccine trials. The results will be linked here as soon as they are published.
STUDY 1: EU-COVAT-1 AGED



A Multinational, Phase 2, Randomised, Adaptive Protocol to Evaluate Immunogenicity and Reactogenicity of Different COVID-19 Vaccines Administration in Older Adults (≥75) Already Vaccinated Against SARS-CoV-2
Lead: Oliver Cornely, Institution/Sponsor: UHC, Germany
Details:
https://clinicaltrials.gov/ct2/show/NCT05160766?cond=Covat+aged&draw=2&rank=1
Publications:
STUDY 2: EU-COVAT-2 BOOSTAVAC


An International Multicentre, Phase 2, Randomised, Adaptive Protocol to determine the need for, optimal timing of and immunogenicity of administering a booster mRNA vaccination dose against SARS-CoV-2 in the general population (18+ years) already vaccinated against SARS-CoV-2
Lead: Patrick Mallon, Institution/Sponsor: NUID UCD, Ireland
EudraCT-number: 2021-004889-35
Details:
https://twitter.com/vaccelerate_eu/status/1503688207506063360
https://www.clinicaltrialsregister.eu/ctr-search/trial/2021-004889-35/IE
STUDY 3: EU-COVPT-1 COVACC



A Phase 2, Comparative Randomised Trial to Evaluate the Impact of Reduced COVID‐19 mRNA Vaccination Regimen on Immunological Responses and Reactogenicity in Paediatric Subjects with Prior SARS‐CoV‐2 Immunity
Lead: Patricia Bruijning-Verhagen, Institution/Sponsor: UMCU, Netherlands
EudraCT number: 2021-005043-71


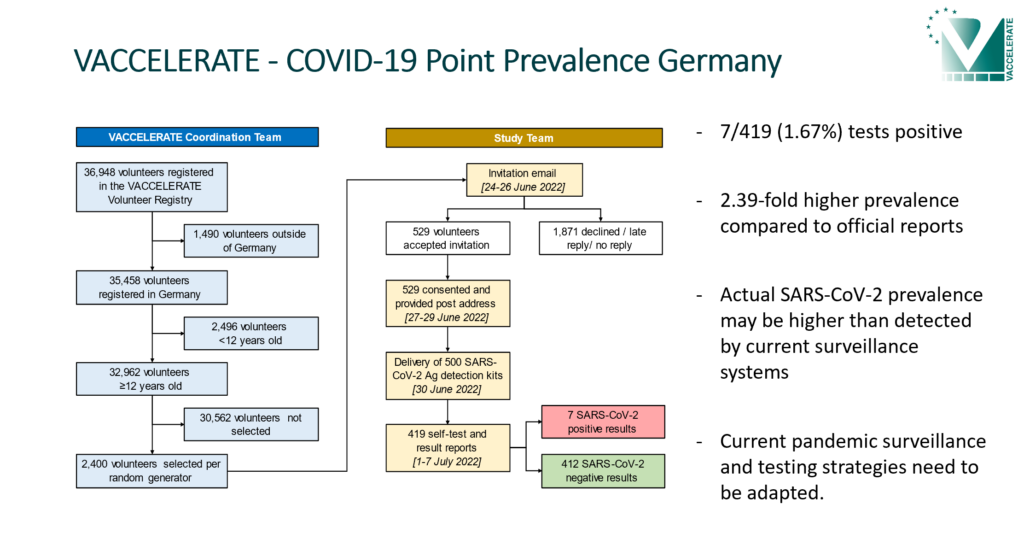
COVID-19 Point Prevalence Germany
VACCELERATE Mpox Initiatives
All information on the VACCELERATE mpox initiatives including the mpox standard, ooperations and publications can be found here: https://vaccelerate.eu/mpox/
Press Releases and Articles on the VACCELERATE Clinical Studies


Publication Video: https://www.youtube.com/watch?v=xTTTNcENoSc
CECAD Newsletter 01/2022
CECAD Excellent in Aging Research
Cluster of Excellence at the University of Cologne

Learn more about the involvement of LAB-Net, the Central Laboratory at the University of Antwerp, in AGED and BOOSTAVAC: https://www.combacte.com/news/lab-net-highly-involved-aged-boostavac/

VACCELERATE Academy
Within VACCELERATE we offer several training courses for the VACCELERATE network, organised through our partners and focusing on various topics concerning (vaccine) trial research.
In the VACCELERATE Education Programme, courses have been identified that are suitable for different study team roles. Some of these courses are required in order to participate in VACCELERATE studies and other courses are recommended for further learning if this is of interest to the participant. Which courses are required for participation differs per study team role.
You can find more information on the courses here.

The COVID-NMA initiative – A living mapping and living systematic review of Covid-19 trials
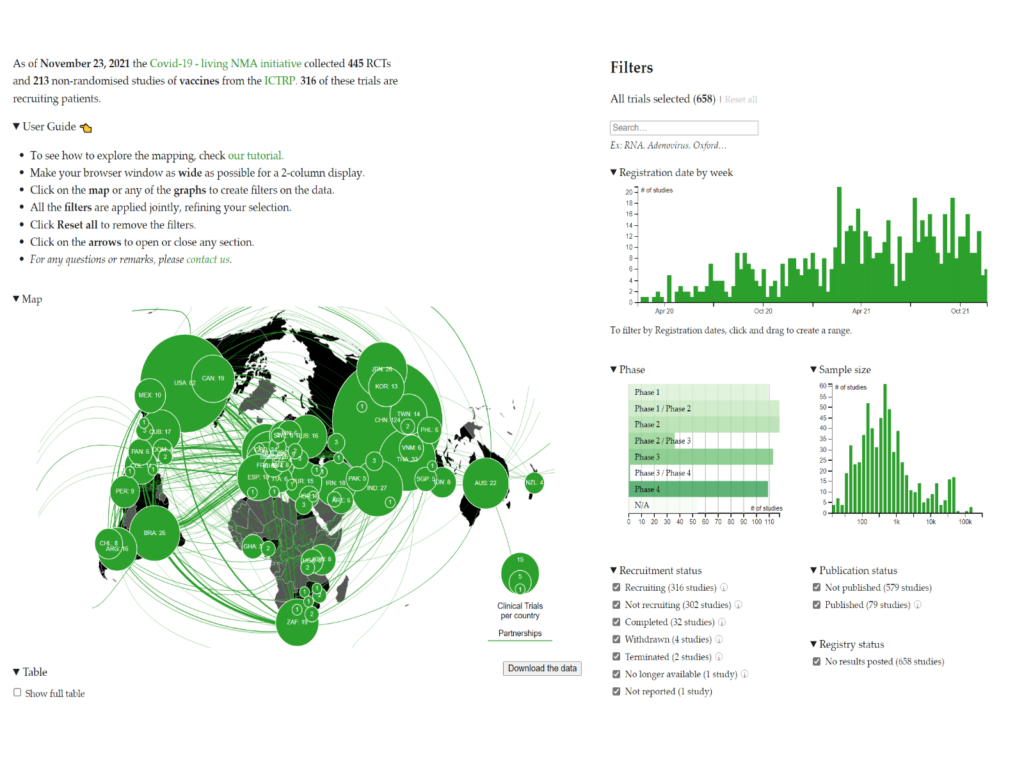
Launched in March 2020, COVID-NMA is an international initiative working in conjunction with the World Health Organization (WHO), led by a team of researchers from Cochrane and other institutions (Université de Paris, Inserm, CNRS, Centre for Evidence-Based Medicine Odense (CEBMO), University of Southern Denmark, Odense University Hospital, Epistemonkos Foundation, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, University of Milan).
The mission is to inform research planning by producing an up-to-date mapping of trial evidence, to inform public health and healthcare decisions by producing relevant, accessible, up-to-date, and trustworthy synthesis of high-quality evidence about the efficacy and safety of interventions for the prevention or treatment of COVID-19 and to improve research value by producing a living monitoring of trial planning, conduct, and reporting and implementing interventions to transform research practices.
For more information: https://covid-nma.com/vaccines/mapping/

