VACCELERATE Site Network
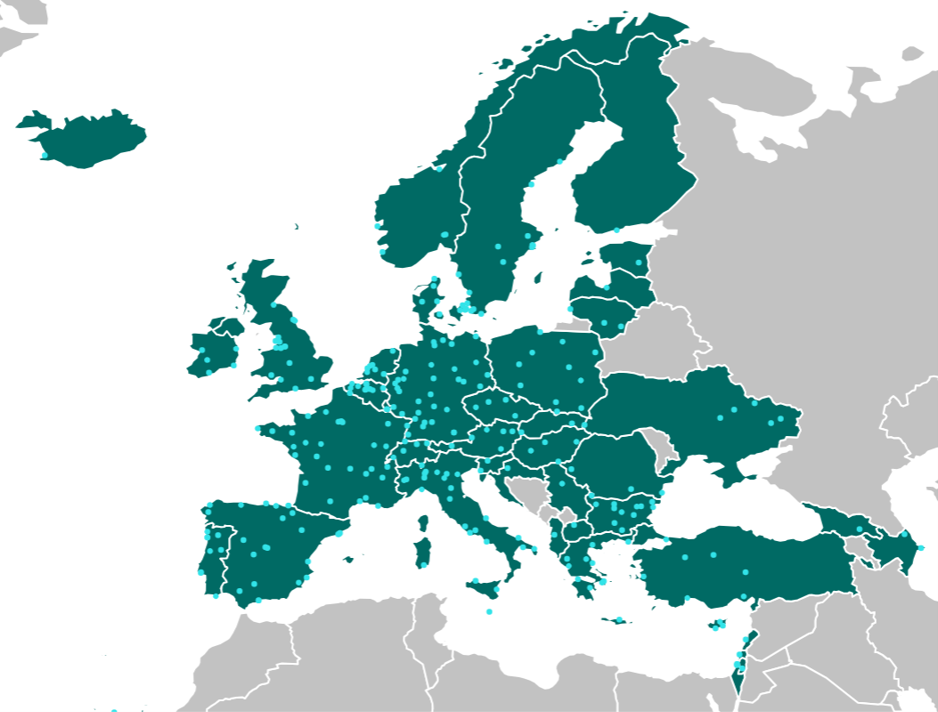
525 clinical trial sites from 57 countries have joined the Site Network so far. The majority is either located in the EU or associated with the H2020 programme.
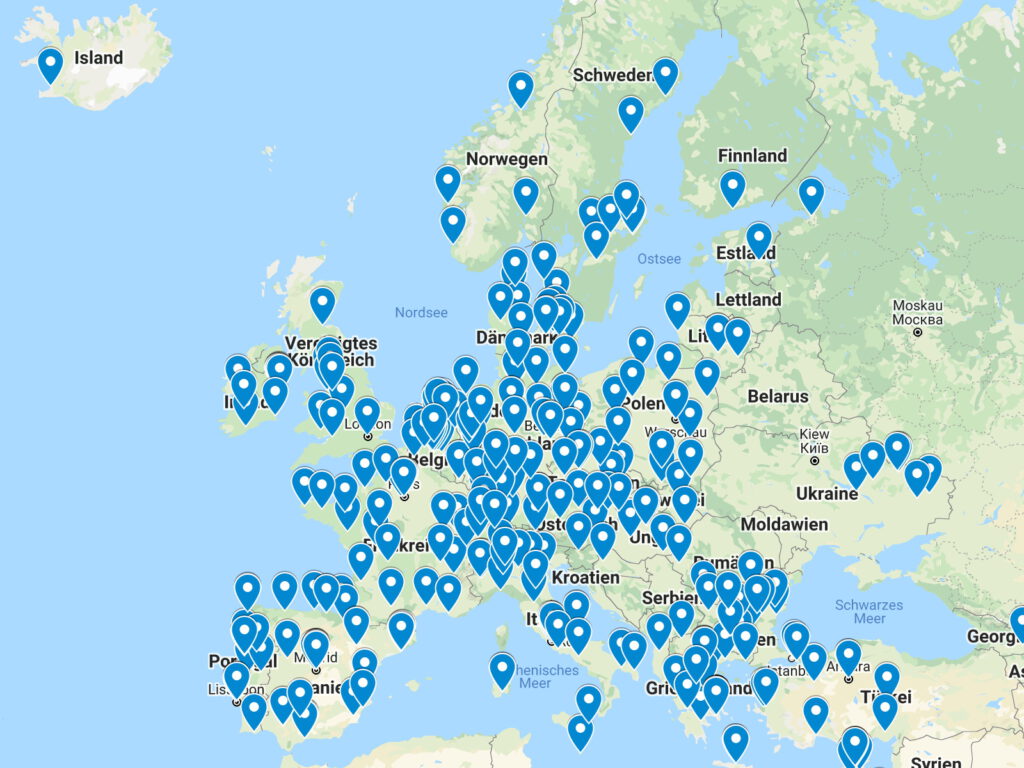
Site Network laboratories
REGISTER NOW!

Become one of our more than 500 registered trial sites and register today!
All you need to do is send an email to trialsites@vaccelerate.eu including:
- Name of your site
- Postal address
- Full name of a contact person
- Contact email address

What is the VACCELERATE Site Network?
The VACCELERATE Site Network is a platform for experienced clinical trial sites interested in conducting (vaccine) studies. Clinical trial sites register with the network database by answering a short site questionnaire. Any clinical trial site from the European Union and neighbouring countries can register.
The VACCELERATE Site Network is managed by the Clinical Trial Infrastructure of the German Center for Infection Research (DZIF) at the University of Cologne.
What does the Site Network seek to accomplish?
VACCELERATE's site network aims to accelerate clinical development for all populations and risk groups. The network database allows for a Europe-wide mapping of experienced clinical trial sites interested in conducting (vaccine) trials.
The Site Network uses its database to put individual sites in contact with clinical trial sponsors, matching the characteristics of each site with the specific requirements of an individual trial.
It acts as an information broker or matchmaker between interested sites and sponsors, it is not a governing or management body for trial conduct. The network interacts with established research networks but also with clinical trial sites not affiliated with a network.
The network thereby intends to boost the conduct of clinical trials of any scale across country borders in specific populations and risk groups that are not the focus of ongoing phase III trials. There is no upfront limitation on study design: Studies may be head-to-head interventional or observational, on short-term or long-term outcomes, focusing on very small populations or else. Any sponsor from academia, small or medium enterprises (SME), biotech or pharmaceutical industry is welcome to contact the Site Network. While the Network was originally mainly designed to roll out vaccine trials, it is now also used for indications beyond.
What information will the Site Network ask for when you register your clinical trial site?
- Name and type of institution, contact details
- Affiliation with infectious disease networks
- Prior clinical trial experience
- Main area of expertise, e.g. disease or indication of interest
- Infrastructure available at your site
- Your preferences for studies, e.g. specific study populations, inpatient vs. outpatient setting etc.
- You can also share the contacts of other sites you wish to recommend for the Site Network

VIDEO on the VACCELERATE Site Network: